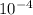Chemistry, 15.07.2019 19:00 lerasteidl
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) determine the direction of reaction if 1.00 m nocl is combined with 0.500 m no and 0.500 m cl2 in a sealed flask. a.)no reaction occurs. b.)reaction proceeds to the right toward the products. c.)reaction proceeds to the left toward the reactants. d.)reaction proceeds to the left toward the products.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) deter...
Questions



Mathematics, 18.08.2019 14:30




Mathematics, 18.08.2019 14:30


History, 18.08.2019 14:30

Geography, 18.08.2019 14:30


Social Studies, 18.08.2019 14:30

History, 18.08.2019 14:30

Mathematics, 18.08.2019 14:30


History, 18.08.2019 14:30

Mathematics, 18.08.2019 14:30

Social Studies, 18.08.2019 14:30

English, 18.08.2019 14:30

Social Studies, 18.08.2019 14:30

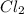
![Q = \frac{[ NO]^{2}. [Cl_{2} ]}{[ NOCl ]^{2}}](/tpl/images/1058/5527/aa67c.png)