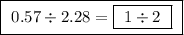Chemistry, 15.07.2019 17:30 Nanamoney5385
Achemist finds that 30.82 g of nitrogen will react with 17.60 g, 35.20 g, 70.40 g, or 88.00 g of oxygen to form four different compounds. calculate the mass of oxygen per gram of nitrogen in each compound.


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
Achemist finds that 30.82 g of nitrogen will react with 17.60 g, 35.20 g, 70.40 g, or 88.00 g of oxy...
Questions


History, 24.01.2021 02:20





Mathematics, 24.01.2021 02:20

Chemistry, 24.01.2021 02:20

Mathematics, 24.01.2021 02:20

Mathematics, 24.01.2021 02:20

Biology, 24.01.2021 02:20




Health, 24.01.2021 02:20


Advanced Placement (AP), 24.01.2021 02:20



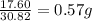 of oxygen
of oxygen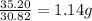 of oxygen
of oxygen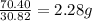 of oxygen
of oxygen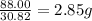 of oxygen
of oxygen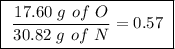
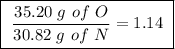
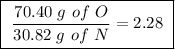
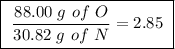
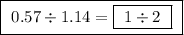 Between the second and third compounds:
Between the second and third compounds: 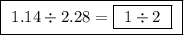 Between the first and third compounds:
Between the first and third compounds: