
Chemistry, 15.07.2019 17:00 ineedhelp2285
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o = 212 kj/mol for the reaction. for this calculation, use the value r = 8.3144 j/k·mol. nio(s) ⇌ ni(s) + 1 2 o2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o = 212 kj/mol for the r...
Questions

Mathematics, 14.06.2021 21:00


Mathematics, 14.06.2021 21:00

Biology, 14.06.2021 21:00

French, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00

English, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00


Mathematics, 14.06.2021 21:00

Spanish, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00


English, 14.06.2021 21:00


Mathematics, 14.06.2021 21:00

Mathematics, 14.06.2021 21:00

Chemistry, 14.06.2021 21:00

Health, 14.06.2021 21:00

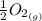 We can find out the pressure on oxygen by using the equation of gibb's free energy with remainder quotient which is, ΔG = ΔG° +RT lnQ
We can find out the pressure on oxygen by using the equation of gibb's free energy with remainder quotient which is, ΔG = ΔG° +RT lnQ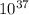
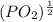 So, (1.51 X
So, (1.51 X  = 3.87 X
= 3.87 X 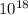
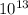 atm
atm

