
Chemistry, 15.07.2019 15:30 kikidsgn123
It takes 238./kjmol to break a carbon-iodine single bond. calculate the maximum wavelength of light for which a carbon-iodine single bond could be broken by absorbing a single photon. round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
It takes 238./kjmol to break a carbon-iodine single bond. calculate the maximum wavelength of light...
Questions


History, 05.09.2019 19:30




English, 05.09.2019 19:30







History, 05.09.2019 19:30


Computers and Technology, 05.09.2019 19:30


English, 05.09.2019 19:30


Mathematics, 05.09.2019 19:30

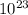 ) = 3.953
) = 3.953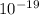 J
J 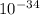 Js X 2.9979
Js X 2.9979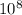 m/s) / (3.953
m/s) / (3.953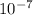 m
m
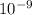 m
m


