
Chemistry, 15.07.2019 14:30 alannadiaz1
It takes 167 s for an unknown gas to effuse through a porous wall and 99 s for the same volume of n2 gas to effuse at the same temperature and pressure. what is the molar mass of the unknown gas?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
It takes 167 s for an unknown gas to effuse through a porous wall and 99 s for the same volume of n2...
Questions

Mathematics, 22.11.2020 04:40

History, 22.11.2020 04:40

Mathematics, 22.11.2020 04:40

History, 22.11.2020 04:40




Mathematics, 22.11.2020 04:40

Mathematics, 22.11.2020 04:40

History, 22.11.2020 04:40




French, 22.11.2020 04:40





Mathematics, 22.11.2020 04:50

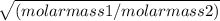
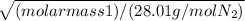 ]
]


