
Chemistry, 13.10.2019 09:30 hannahcolleen4895
Fill in the blanks:
1. during a chemical reaction, the sum of the of the reactants and products remain unchanged.
2. in a pure chemical compound, elements are always present in a proportion by mass.
3. clusters of atoms that act as an ion are called ions.
4. in ionic compounds, the charge on each ion is used to determine the the compound.
5. the avogadro constant is defined as the number of atoms in exactly of carbon-12
6. mass of 1 mole of a substance is called its
7. the abbreviation used for length names of elements are termed as their
8. a chemical formula is also known as a
9. those ions which are formed from single atoms are called
10. ionic compounds are formed by the combination between an
11. the valency of an ion is to the charge on the ion.
12. mole is a link between the and
13. the si unit of amount of a substance is
true / false:
1. formula mass of na2o is 62 amu.
2. those particles which have more or less electrons than the normal atoms are called ions
.
3. formula for sulphur dioxide is so3.
4. molar mass of ethyne (c2h2) is 26 g/mol.
5. 22 gm of co2 consists of 1 mole.
6. number of molecules in 32 gram of oxygen is 6.02 x 1023.
7. water is an atom.
8. formula for sulphur dioxide is so2.
9. clusters of atoms that act as an ion is called lopyatomic ion.
10. mass of 1 mole of a substance is called its formula mass.
11. in a pure chemical compound, elements are always present in a definite proportion by mass.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
Fill in the blanks:
1. during a chemical reaction, the sum of the of the reactants and...
1. during a chemical reaction, the sum of the of the reactants and...
Questions




Computers and Technology, 13.08.2019 03:20


Computers and Technology, 13.08.2019 03:20














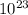 is defined as the number of atoms in exactly 12 g of carbon-12
is defined as the number of atoms in exactly 12 g of carbon-12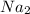 O is 62 amu True
O is 62 amu True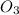 . False
. False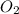 as the name di suggests two molecules.
as the name di suggests two molecules.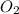 contains 1 C atom and 2 O atoms (12) + (16 X 2) = 44
contains 1 C atom and 2 O atoms (12) + (16 X 2) = 44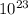 . False
. False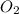 true.
true.

