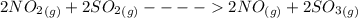Sulfuric acid was once produced through the reaction of sulfur trioxide with water. sulfur trioxide can form through the reaction of sulfur dioxide and oxygen gas. when nitrogen monoxide gas is added to the system, the reaction speeds up significantly because it proceeds through the following steps: 2no(g)+o2(> 2no2(g) 2no2(g)+> 2no(g)+2so3(g) identify the catalyst in this reaction, explain how you know it is the catalyst, and describe how it increases the rate of the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Sulfuric acid was once produced through the reaction of sulfur trioxide with water. sulfur trioxide...
Questions



Biology, 19.10.2021 01:00

History, 19.10.2021 01:00

Mathematics, 19.10.2021 01:00



History, 19.10.2021 01:00


Mathematics, 19.10.2021 01:00





Mathematics, 19.10.2021 01:00

History, 19.10.2021 01:00


SAT, 19.10.2021 01:00