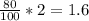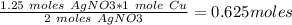Chemistry, 15.07.2019 10:30 Andrebutrus
#3.) hydrogen and oxygen react to produce water, according to the reaction 2h2 (g) + o2 (g) → 2h2o (l). if 3 moles of hydrogen react with 2 moles of oxygen and the yield in the reaction is 80%, how many moles of water are obtained? select one: a. 2 moles b. 2.4 moles c. 2.7 moles d. 3 moles#4.) which of the following is true about the total number of reactants and the total number of products in the reaction shown below? c5h12(l) + 8o2(g) → 5co2(g) + 6h2o(g)select one: a. 9 moles of reactants chemically change into 11 moles of product. b. 9 grams of reactants chemically change into 11 grams of product. c. 9 liters of reactants chemically change into 11 liters of product. d. 9 atoms of reactants chemically change into 11 atoms of product.#9.) how many moles of cu are needed to react with 1.23 moles of agno3? cu + 2agno3 → cu(no3)2 + 2agselect one: a. 0.62b. 2.5c. 3.51d. 0.55

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
#3.) hydrogen and oxygen react to produce water, according to the reaction 2h2 (g) + o2 (g) → 2h2o (...
Questions



Advanced Placement (AP), 28.06.2019 00:30

English, 28.06.2019 00:30

Spanish, 28.06.2019 00:30



Mathematics, 28.06.2019 00:30



Chemistry, 28.06.2019 00:30

Biology, 28.06.2019 00:30


Mathematics, 28.06.2019 00:30

Mathematics, 28.06.2019 00:30


History, 28.06.2019 00:30

Mathematics, 28.06.2019 00:30


History, 28.06.2019 00:30