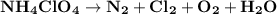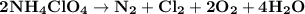Chemistry, 15.07.2019 10:00 angelaencinas90
Ammonium perchlorate nh4clo4 is a powerful solid rocket fuel, used in the space shuttle boosters. it decomposes into nitrogen n2 gas, chlorine cl2 gas, oxygen o2 gas and water vapor, releasing a great deal of energy. calculate the moles of ammonium perchlorate needed to produce 0.10mol of chlorine. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Ammonium perchlorate nh4clo4 is a powerful solid rocket fuel, used in the space shuttle boosters. it...
Questions

Biology, 01.02.2020 07:44


History, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44



English, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44

Chemistry, 01.02.2020 07:44





Health, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44


Health, 01.02.2020 07:44