

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
How many moles of ag can be produced if 350. g of cu are reacted with excess agno3 according to the...
Questions





Mathematics, 27.10.2019 06:43

Business, 27.10.2019 06:43



Spanish, 27.10.2019 06:43

Physics, 27.10.2019 06:43


Mathematics, 27.10.2019 06:43




Mathematics, 27.10.2019 06:43


Mathematics, 27.10.2019 06:43

Mathematics, 27.10.2019 06:43

Mathematics, 27.10.2019 06:43

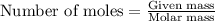
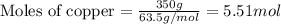
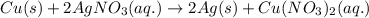
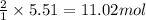 of silver.
of silver.

