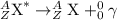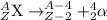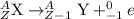After a radioactive atom decays, it is the same element that it was before with no measurable change in mass. which kind of decay has occurred, and how do you know? a) alpha decay because alpha particles have no mass b) beta decay because this kind of decay cannot change one element into another c) alpha decay because it creates a new isotope of the same element d) gamma decay because photons have no mass

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1


Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
After a radioactive atom decays, it is the same element that it was before with no measurable change...
Questions


Geography, 06.10.2020 14:01

Geography, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01





English, 06.10.2020 14:01

Chemistry, 06.10.2020 14:01




Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01