

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Part a.1. the mass of kio3 used to prepare the solution was measured to be 0.585 g instead of the ca...
Questions

Social Studies, 04.03.2021 18:50


Chemistry, 04.03.2021 18:50




Mathematics, 04.03.2021 18:50


Business, 04.03.2021 18:50


Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50

Computers and Technology, 04.03.2021 18:50


Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50

Geography, 04.03.2021 18:50


World Languages, 04.03.2021 18:50

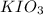 .
.

