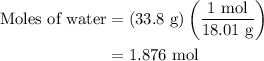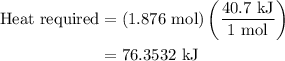Chemistry, 15.07.2019 02:30 maggie123456751
How much heat is required to vaporize 33.8 g of water at 100 ∘c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2


Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
How much heat is required to vaporize 33.8 g of water at 100 ∘c?...
Questions

Mathematics, 28.01.2021 18:10

Mathematics, 28.01.2021 18:10

English, 28.01.2021 18:10

Arts, 28.01.2021 18:10



Mathematics, 28.01.2021 18:10



English, 28.01.2021 18:10


Mathematics, 28.01.2021 18:10

English, 28.01.2021 18:10

Biology, 28.01.2021 18:10





Mathematics, 28.01.2021 18:10

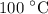 is
is 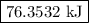 .
.
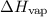 . Its unit is kJ/mol or J/mol.
. Its unit is kJ/mol or J/mol.
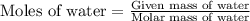 …… (1)
…… (1)