

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
Assuming the volume of the insoluble substance to be 92.5 ml and the mass to be 693.8 g, what is the...
Questions

History, 11.10.2019 16:30



Health, 11.10.2019 16:30

Mathematics, 11.10.2019 16:30

Mathematics, 11.10.2019 16:30

Computers and Technology, 11.10.2019 16:30





Mathematics, 11.10.2019 16:30




Social Studies, 11.10.2019 16:30




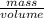
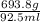 = 7.50 g/ml
= 7.50 g/ml

