
Chemistry, 14.07.2019 23:30 hernandez48tur
8. given the balanced chemical equation br2 + 2 nai -> 2 nabr + i2 how many moles of sodium bromide (nabr) could be produced from 0.172 mol of bromine (br2)?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

You know the right answer?
8. given the balanced chemical equation br2 + 2 nai -> 2 nabr + i2 how many moles of sodium brom...
Questions

Arts, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30

English, 21.01.2021 18:30

History, 21.01.2021 18:30


History, 21.01.2021 18:30


English, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30


Mathematics, 21.01.2021 18:30



Social Studies, 21.01.2021 18:30


History, 21.01.2021 18:30




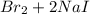 →
→ 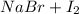
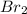 ), so it has a ratio of 1:1 with sodium bromide (
), so it has a ratio of 1:1 with sodium bromide (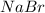 ).
).

