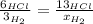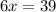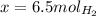Chemistry, 14.07.2019 22:30 maryalice2002
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can be formed? 2 al + 6hcl → 2 alcl3 + 3 h2 select one: a. al is the limiting reactant, 9.0 mol h2 can be formed b. hcl is the limiting reactant, 6.5 mol h2 can be formed c. al is the limiting reactant, 6.0 mol h2 can be formed d. hcl is the limiting reactant, 4.3 mol h2 can be formed

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can b...
Questions

Mathematics, 28.12.2019 11:31

Biology, 28.12.2019 11:31

Mathematics, 28.12.2019 11:31

Mathematics, 28.12.2019 11:31



Spanish, 28.12.2019 11:31





Mathematics, 28.12.2019 11:31



Mathematics, 28.12.2019 11:31

History, 28.12.2019 11:31

Mathematics, 28.12.2019 11:31

English, 28.12.2019 11:31


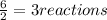
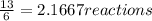
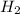 that are produced with 13 moles of HCl.
that are produced with 13 moles of HCl.