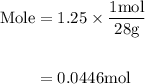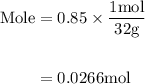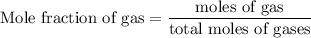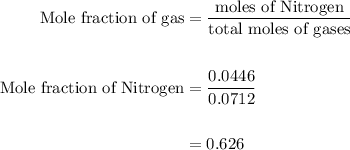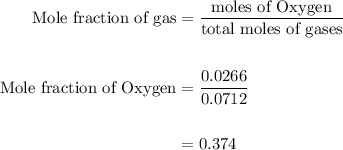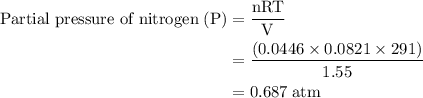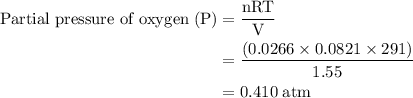Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Agas mixture contains 1.25 g n2 and 0.85 g o2 in a 1.55 l c ontainer at 18 °c. calculate the mole fr...
Questions

Mathematics, 23.04.2021 04:30

History, 23.04.2021 04:30


Chemistry, 23.04.2021 04:30


Business, 23.04.2021 04:30







Mathematics, 23.04.2021 04:30



Mathematics, 23.04.2021 04:30

Mathematics, 23.04.2021 04:30

Mathematics, 23.04.2021 04:30

Mathematics, 23.04.2021 04:30

Mathematics, 23.04.2021 04:30