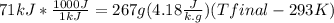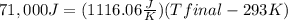Chemistry, 14.07.2019 18:00 jasminortega2002
23 grams of sodium reacts with 244 cm3 of water that is initially at 293 k. it produces an enthalpy change of 71 kj. what is the final temperature of the water? the specific heat capacity of water is 4.18 j/k g.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
23 grams of sodium reacts with 244 cm3 of water that is initially at 293 k. it produces an enthalpy...
Questions

Mathematics, 30.07.2021 07:50




Mathematics, 30.07.2021 07:50

Mathematics, 30.07.2021 07:50


Computers and Technology, 30.07.2021 07:50

Mathematics, 30.07.2021 07:50

Biology, 30.07.2021 07:50



English, 30.07.2021 07:50



Mathematics, 30.07.2021 07:50

Computers and Technology, 30.07.2021 07:50


Mathematics, 30.07.2021 07:50

English, 30.07.2021 07:50

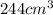
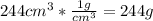
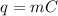 ΔT
ΔT