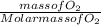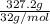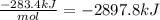Chemistry, 14.07.2019 18:00 AleciaCassidy
Solid sodium peroxide (na2o2) reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas. how much heat is released if 327.2 g of oxygen gas is produced from the reaction of sodium peroxide and water under standard-state conditions?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

You know the right answer?
Solid sodium peroxide (na2o2) reacts with liquid water yielding aqueous sodium hydroxide and oxygen...
Questions


Mathematics, 26.06.2019 13:20


Mathematics, 26.06.2019 13:20



Mathematics, 26.06.2019 13:20

History, 26.06.2019 13:20



Mathematics, 26.06.2019 13:20