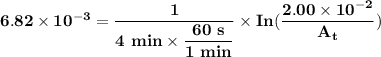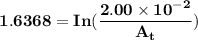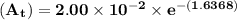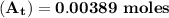Chemistry, 14.07.2019 17:30 guccikathyyy6195
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×10−3 s−1. suppose we start with 2.00×10−2 mol of n2o5(g) in a volume of 2.3 l . you may want to reference (page) section 14.4 while completing this problem. part a how many moles of n2o5 will remain after 4.0 min ? ,

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3


Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×...
Questions

Health, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20


Chemistry, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20


Mathematics, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

World Languages, 08.12.2020 17:20


Mathematics, 08.12.2020 17:20


Chemistry, 08.12.2020 17:20


Mathematics, 08.12.2020 17:20

Arts, 08.12.2020 17:20

English, 08.12.2020 17:20

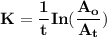
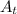 = ???
= ???