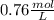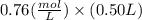Chemistry, 14.07.2019 16:30 thomasmurphy200
The benzoate ion, c6h5coo− is a weak base with kb=1.6×10−10. how many moles of sodium benzoate are present in 0.50 l of a solution of nac6h5coo if the ph is 9.04?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
The benzoate ion, c6h5coo− is a weak base with kb=1.6×10−10. how many moles of sodium benzoate are p...
Questions

Spanish, 28.01.2020 02:31

Mathematics, 28.01.2020 02:31

Physics, 28.01.2020 02:31

Chemistry, 28.01.2020 02:31

English, 28.01.2020 02:31



Chemistry, 28.01.2020 02:31

History, 28.01.2020 02:31





Biology, 28.01.2020 02:31


Business, 28.01.2020 02:31

Health, 28.01.2020 02:31



Health, 28.01.2020 02:31

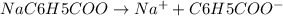
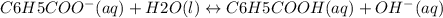
![[OH^{-}] = 10^{^{-pOH}}](/tpl/images/0089/2713/549cf.png)
![[OH^{-}] = 10^{^{-4.96}}](/tpl/images/0089/2713/ff5c3.png)
![[OH^{-}] = 1.1\times 10^{-5}](/tpl/images/0089/2713/97cf5.png)
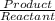
![kb =\frac{[C6H5COOH][OH^{-}]}{[C6H5COO^{-}]}](/tpl/images/0089/2713/6f928.png)
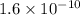
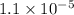 and value of C6H5COOH is equal to OH-
and value of C6H5COOH is equal to OH-![1.6\times 10^{-10} = \frac{[1.1\times 10^{-5}][1.1\times 10^{-5}]}{[C6H5COO^{-}]}](/tpl/images/0089/2713/af234.png)
![[C6H5COO^{-}] = \frac{[1.1\times 10^{-5}][1.1\times 10^{-5}]}{1.6\times 10^{-10}}](/tpl/images/0089/2713/c4847.png)
![[C6H5COO^{-}] = 0.76 M](/tpl/images/0089/2713/e77f3.png) or
or