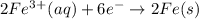Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Use tabulated half-cell potentials to calculate δg∘rxn for each of the following reactions at 25 ∘c....
Questions

Geography, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01


History, 02.10.2020 15:01

English, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01

Social Studies, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01




English, 02.10.2020 15:01

Physics, 02.10.2020 15:01

Geography, 02.10.2020 15:01


 for the reaction is -58 kJ/mol.
for the reaction is -58 kJ/mol.
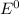 = 0.14 V
= 0.14 V