Chemistry, 14.07.2019 13:30 meganjackson155
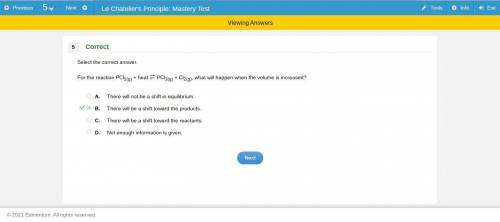
For the reaction pcl5(g) + heat pcl3(g) + cl2(g), what will happen when the volume is increased? a. there will not be a shift in equilibrium. b. there will be a shift toward the products. c. there will be a shift toward the reactants. d. not enough information is given. if you can figure out the answer, you.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

You know the right answer?
For the reaction pcl5(g) + heat pcl3(g) + cl2(g), what will happen when the volume is increased? a....
Questions

Computers and Technology, 14.12.2019 03:31









Computers and Technology, 14.12.2019 03:31