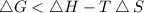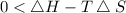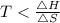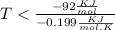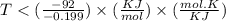Chemistry, 14.07.2019 12:30 natishtaylor1p8dirz
At which temperature would a reaction with h -92 kj/mol, s -0.199 kj/(mol-k) be spontaneous? a.600k b.500k c.400k d.700k

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
At which temperature would a reaction with h -92 kj/mol, s -0.199 kj/(mol-k) be spontaneous? a.600k...
Questions




Computers and Technology, 27.03.2020 03:07

Computers and Technology, 27.03.2020 03:07


Health, 27.03.2020 03:07

Mathematics, 27.03.2020 03:07




Mathematics, 27.03.2020 03:07





English, 27.03.2020 03:08

Mathematics, 27.03.2020 03:08

Mathematics, 27.03.2020 03:08

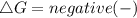
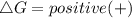
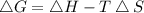
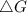 should be negative , so the above formula can be written as :
should be negative , so the above formula can be written as :