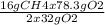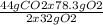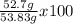Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 and 78.3g o2 to react. when the reaction is finished, the chemist collects 52.7g co2. determine the limiting reagent, theoretical yield, and percent yield for the reaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 an...
Questions

Mathematics, 27.02.2020 07:39

Mathematics, 27.02.2020 07:39

History, 27.02.2020 07:39

Mathematics, 27.02.2020 07:39


Mathematics, 27.02.2020 07:39




Mathematics, 27.02.2020 07:40

Mathematics, 27.02.2020 07:40

Mathematics, 27.02.2020 07:40


Mathematics, 27.02.2020 07:40

History, 27.02.2020 07:41

Mathematics, 27.02.2020 07:41

Mathematics, 27.02.2020 07:41

Mathematics, 27.02.2020 07:41