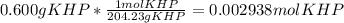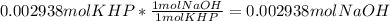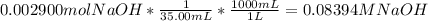Chemistry, 14.07.2019 12:00 potatogirl6811
Reagent grade potassium hydrogen phthalate (khp, mass 204.23g/mole) is a high molecular weight, stable, monoprotic solid acid. it is commonly used for standardizing sodium hydroxide solutions. what concentration of sodium hydroxide solution would be needed to titration 0.6000g of khp so that the volume of naoh needed is 35.00ml

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 10:30
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
Reagent grade potassium hydrogen phthalate (khp, mass 204.23g/mole) is a high molecular weight, stab...
Questions

Biology, 28.07.2019 01:30


Mathematics, 28.07.2019 01:30


Arts, 28.07.2019 01:30

History, 28.07.2019 01:30

History, 28.07.2019 01:30



Chemistry, 28.07.2019 01:30


Mathematics, 28.07.2019 01:30



Spanish, 28.07.2019 01:30