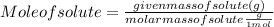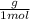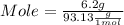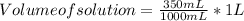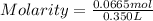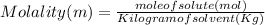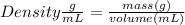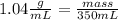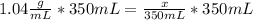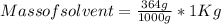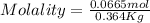Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Astudent dissolves 6.2g of aniline c6h5nh2 in 350.ml of a solvent with a density of 1.04/gml . the s...
Questions





Chemistry, 14.01.2021 02:20


Mathematics, 14.01.2021 02:20

Social Studies, 14.01.2021 02:20



Mathematics, 14.01.2021 02:20


Mathematics, 14.01.2021 02:20

History, 14.01.2021 02:20

German, 14.01.2021 02:20

Mathematics, 14.01.2021 02:20

French, 14.01.2021 02:20


Chemistry, 14.01.2021 02:20

Arts, 14.01.2021 02:20

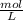 and Molality = 0.18
and Molality = 0.18 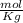 .
.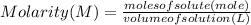 \
\