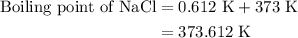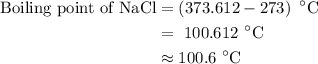Chemistry, 14.07.2019 04:30 Connor20000006
Estimate the boiling point of a 0.6 molal solution of nacl in water. the boiling point elevation constant of water is 0.51 k/molal. give your answer in °c. 99.7˚c 373.45 ˚c 373.75 ˚c 100.6 ˚c 100.3˚c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Estimate the boiling point of a 0.6 molal solution of nacl in water. the boiling point elevation con...
Questions

Geography, 03.02.2020 10:48



Chemistry, 03.02.2020 10:48


Mathematics, 03.02.2020 10:48

Mathematics, 03.02.2020 10:48


English, 03.02.2020 10:48

Mathematics, 03.02.2020 10:48

English, 03.02.2020 10:48


Mathematics, 03.02.2020 10:48

Mathematics, 03.02.2020 10:48


Mathematics, 03.02.2020 10:48

Computers and Technology, 03.02.2020 10:48



Mathematics, 03.02.2020 10:48

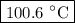 .
.
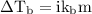 …… (1)
…… (1)
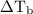 is boiling point elevation.
is boiling point elevation.
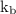 is boiling point elevation constant.
is boiling point elevation constant.
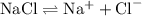
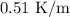 for
for 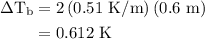
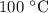 . It is to be converted into Kelvin with help of following conversion factor:
. It is to be converted into Kelvin with help of following conversion factor:
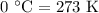
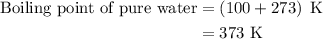
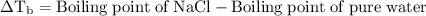 …… (2)
…… (2)
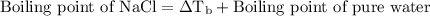 …… (3)
…… (3)