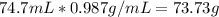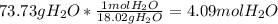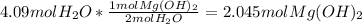Chemistry, 14.07.2019 04:30 haileyjones732
How many moles of magnesium hydroxide are needed to produce 74.7 milliliters of water, if the density of water is 0.987 g/ml? show all steps of your calculations as well as the final answer. hno3 + mg(oh)2 → h2o + mg(no3)2?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
How many moles of magnesium hydroxide are needed to produce 74.7 milliliters of water, if the densit...
Questions


Mathematics, 12.02.2021 07:10




Mathematics, 12.02.2021 07:10

English, 12.02.2021 07:10

Mathematics, 12.02.2021 07:10


Mathematics, 12.02.2021 07:10









Social Studies, 12.02.2021 07:10