
Chemistry, 14.07.2019 04:00 gilbert325
If 3.5 moles of nitrogen monoxide (no) react with 6.0 moles of oxygen gas (o2), how many moles of the product can be formed and how many moles of the excess reactant will be left over when the reaction is complete? show all of your work. unbalanced equation: no + o2 “yields”/ no2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
If 3.5 moles of nitrogen monoxide (no) react with 6.0 moles of oxygen gas (o2), how many moles of th...
Questions

Arts, 28.05.2021 08:00

Mathematics, 28.05.2021 08:00

Mathematics, 28.05.2021 08:00


Mathematics, 28.05.2021 08:00

Mathematics, 28.05.2021 08:00


Mathematics, 28.05.2021 08:00


Mathematics, 28.05.2021 08:00

Mathematics, 28.05.2021 08:00


Engineering, 28.05.2021 08:00

Chemistry, 28.05.2021 08:00

Mathematics, 28.05.2021 08:00

English, 28.05.2021 08:00


Geography, 28.05.2021 08:00


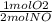 x 3.5 mol NO
x 3.5 mol NO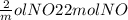 x 3.5 mol NO
x 3.5 mol NO

