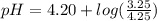Do parts a, b and c a. calculate the ph of 100 ml of an aqueous solution of 0.05 m benzoic acid and 0.025 m benzoate ion. the ka of benzoic acid is 6.3 x 10-5 at 25 ℃. b. calculate the new ph of the solution in part a if you add 10.0 ml of 0.100 m hcl solution. c. calculate the new ph of the solution in part a if you add 15.0 ml of 0.050 m naoh solution.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Do parts a, b and c a. calculate the ph of 100 ml of an aqueous solution of 0.05 m benzoic acid and...
Questions


Advanced Placement (AP), 18.09.2021 21:10

Mathematics, 18.09.2021 21:10

Biology, 18.09.2021 21:10



English, 18.09.2021 21:10





Mathematics, 18.09.2021 21:10

Mathematics, 18.09.2021 21:10

Mathematics, 18.09.2021 21:10

History, 18.09.2021 21:10

Mathematics, 18.09.2021 21:10

Mathematics, 18.09.2021 21:10


Mathematics, 18.09.2021 21:10

Geography, 18.09.2021 21:10

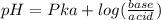
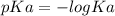
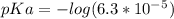
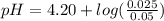
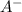 .
.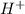 added = 0.100(10.0) = 1
added = 0.100(10.0) = 1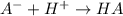
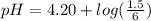
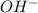 added to the original buffer = 0.05(15.0) = 0.75
added to the original buffer = 0.05(15.0) = 0.75