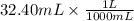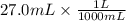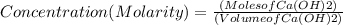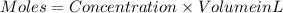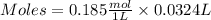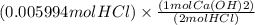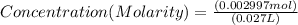Chemistry, 14.07.2019 02:30 fespinoza019
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what is the concentration of the ca(oh)2 solution? show all of the work needed to solve this problem. (2 points) ca(oh)2 + 2hcl yields 2h2 o + cacl2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
If 27.0 ml of ca(oh)2 with an unknown concentration is neutralized by 32.40 ml of 0.185 m hcl, what...
Questions


History, 14.01.2021 02:40

Mathematics, 14.01.2021 02:40

Mathematics, 14.01.2021 02:40

Mathematics, 14.01.2021 02:40




Mathematics, 14.01.2021 02:40


Mathematics, 14.01.2021 02:40

Mathematics, 14.01.2021 02:40


Mathematics, 14.01.2021 02:40

Health, 14.01.2021 02:40

Mathematics, 14.01.2021 02:40



Computers and Technology, 14.01.2021 02:40

Arts, 14.01.2021 02:40