
Chemistry, 14.07.2019 02:00 gameranonymous266
Chem consider the reaction: ? li(s)+? n2(> ? li3n(s) calculate the mass of lithium nitride formed from 33.6524g of nitrogen gas and 58.7032g lithium

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Chem consider the reaction: ? li(s)+? n2(> ? li3n(s) calculate the mass of lithium nitride for...
Questions

Mathematics, 26.06.2019 08:10

History, 26.06.2019 08:10

Chemistry, 26.06.2019 08:10

Mathematics, 26.06.2019 08:10

Chemistry, 26.06.2019 08:10

Mathematics, 26.06.2019 08:10



Mathematics, 26.06.2019 08:10

History, 26.06.2019 08:10

Mathematics, 26.06.2019 08:10

Mathematics, 26.06.2019 08:10

History, 26.06.2019 08:10


Mathematics, 26.06.2019 08:10

Biology, 26.06.2019 08:10

Mathematics, 26.06.2019 08:10

Biology, 26.06.2019 08:10

Mathematics, 26.06.2019 08:10

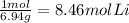
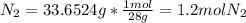
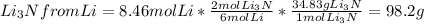
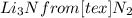 = 1.2 mol
= 1.2 mol 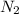 * \frac{2 mol Li_{3}N}{1 mol N2}* \frac{34.83 g Li_{3}N}{1 mol Li_{3}N} = 98.2 g " alt=" N_{2}" /> = 1.2 mol
* \frac{2 mol Li_{3}N}{1 mol N2}* \frac{34.83 g Li_{3}N}{1 mol Li_{3}N} = 98.2 g " alt=" N_{2}" /> = 1.2 mol 

