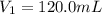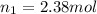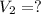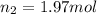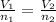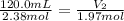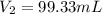Chemistry, 14.07.2019 01:00 alondris3888
If 2.38 mol of a gas has a volume of 120.0 ml, what is the volume of 1.97 mol of the gas at the same temperature and pressure?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 23.06.2019 13:30
Consider this reaction taking place in a closed 2 liter container: 2so2(g) + o2(g) → 2so3(g) if the volume of the container is decreased to 1 liter, what will happen to the equilibrium of the reaction? it will shift left. it will shift right. it will remain constant it will decrease by half
Answers: 3

Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
If 2.38 mol of a gas has a volume of 120.0 ml, what is the volume of 1.97 mol of the gas at the same...
Questions

Mathematics, 04.03.2022 09:50

Mathematics, 04.03.2022 09:50


Social Studies, 04.03.2022 14:00

Geography, 04.03.2022 14:00

Biology, 04.03.2022 14:00







Business, 04.03.2022 14:00



Computers and Technology, 04.03.2022 14:00

Social Studies, 04.03.2022 14:00


Mathematics, 04.03.2022 14:00