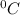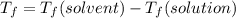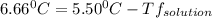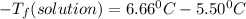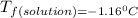Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3


Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Calculate the freezing point of a solution made from 22.0 g of octane (c8h18) dissolved in 148.0 g o...
Questions

Mathematics, 02.04.2021 19:10

Mathematics, 02.04.2021 19:10



English, 02.04.2021 19:10


History, 02.04.2021 19:10

Mathematics, 02.04.2021 19:10




English, 02.04.2021 19:10




English, 02.04.2021 19:10


Mathematics, 02.04.2021 19:10


Biology, 02.04.2021 19:10

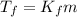
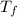 is the change in the freezing point of the solvent.
is the change in the freezing point of the solvent.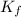 of benzene is 5.12
of benzene is 5.12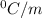
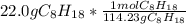 = 0.193 mol
= 0.193 mol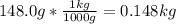
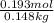
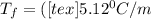 ) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />
) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />