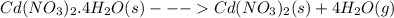Chemistry, 13.07.2019 23:30 eddsworldfrantic
The crystalline hydrate cd(no3)2 ⋅ 4h2o(s) loses water when placed in a large, closed, dry vessel at room temperature: cd(no3)2⋅4h2o(s)→ cd(no3)2(s) 4 h2o(g) this process is spontaneous and δh∘ is positive at room temperature.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
The crystalline hydrate cd(no3)2 ⋅ 4h2o(s) loses water when placed in a large, closed, dry vessel at...
Questions

Mathematics, 10.11.2020 01:30


Physics, 10.11.2020 01:30





Mathematics, 10.11.2020 01:30





English, 10.11.2020 01:30


Mathematics, 10.11.2020 01:30

Mathematics, 10.11.2020 01:30

Mathematics, 10.11.2020 01:30


Biology, 10.11.2020 01:30

English, 10.11.2020 01:30