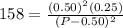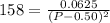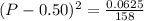Chemistry, 13.07.2019 22:00 elijahdouglass00
Asample of pure no2 gas decomposes at 1000 k 2no2 (g) ↔ 2 no (g) + o2 (g) the constant kp is 158. an analysis shows that the partial pressure of o2 is 0.25 atmospheres at equilibrium. determine the pressure of no and no2.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Asample of pure no2 gas decomposes at 1000 k ...
Questions



Mathematics, 15.07.2020 02:01


Mathematics, 15.07.2020 02:01

English, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01


English, 15.07.2020 02:01


Mathematics, 15.07.2020 02:01

English, 15.07.2020 02:01




Mathematics, 15.07.2020 02:01



Chemistry, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01

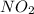 = 0.02 atm.
= 0.02 atm.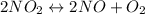
![Kp=(\frac{[NO]^2[O_2]}{[NO_2]^2})](/tpl/images/0086/2744/569f6.png)