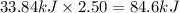Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: what is the enthalpy change when 2.50 mol of nitrogen dioxide decomposes? 13.5 kj of energy released 13.5 kj of energy absorbed 84.6 kj of energy released 84.6 kj of energy absorbed

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2

Chemistry, 23.06.2019 14:30
The hammering on a train track is often heard twice by workers farther down the track; first as the sound travels through the steel and second as the sound travels through the air. this suggests which graph is true?
Answers: 1
You know the right answer?
Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: wh...
Questions

Mathematics, 20.02.2021 05:20


Business, 20.02.2021 05:20




Health, 20.02.2021 05:20

Mathematics, 20.02.2021 05:20


Business, 20.02.2021 05:20

History, 20.02.2021 05:20



Mathematics, 20.02.2021 05:20

Mathematics, 20.02.2021 05:20

Physics, 20.02.2021 05:20

Business, 20.02.2021 05:20


Physics, 20.02.2021 05:20

Mathematics, 20.02.2021 05:20

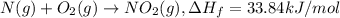
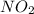 is formed = 33.84 kJ
is formed = 33.84 kJ