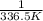Chemistry, 13.07.2019 21:30 thomasg185
The vapor pressure of ethanol is 400. mmhg at 63.5°c. its molar heat of vaporization is 39.3 kj/mol. what is the vapor pressure of ethanol, in mmhg, at 34.9°c? (r = 8.314 j/k • mol)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
The vapor pressure of ethanol is 400. mmhg at 63.5°c. its molar heat of vaporization is 39.3 kj/mol....
Questions

Advanced Placement (AP), 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

English, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01


Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

Social Studies, 10.09.2020 04:01

Biology, 10.09.2020 04:01

Business, 10.09.2020 04:01

Spanish, 10.09.2020 04:01

Mathematics, 10.09.2020 04:01

 -
-