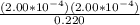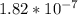Chemistry, 13.07.2019 21:30 ivanyeli4520
The equilibrium concentrations of the reactants and products are [ha] = 0.220 m [h3o ] = 2.00 × 10–4 m [a–] = 2.00 × 10–4 m calculate the ka value for the acid ha.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
The equilibrium concentrations of the reactants and products are [ha] = 0.220 m [h3o ] = 2.00 × 10–4...
Questions


Social Studies, 21.11.2019 04:31



English, 21.11.2019 04:31


Mathematics, 21.11.2019 04:31

History, 21.11.2019 04:31


Biology, 21.11.2019 04:31



History, 21.11.2019 04:31






Social Studies, 21.11.2019 04:31

Biology, 21.11.2019 04:31

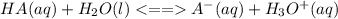
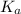 is the equilibrium constant for this equation, which is referred to as the acid dissociation constant.
is the equilibrium constant for this equation, which is referred to as the acid dissociation constant. ![K_{a} = \frac{[H_{3}O^{+}][A^{-}]}{[HA]}](/tpl/images/0086/2201/14aa4.png)