
Chemistry, 13.07.2019 20:00 janshnajdajdnnahjd
How much heat is evolved in converting 1.00 mol of steam at 140.0 ∘c to ice at -55.0 ∘c? the heat capacity of steam is 2.01 j/(g⋅∘c) and of ice is 2.09 j/(g⋅∘c)?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
How much heat is evolved in converting 1.00 mol of steam at 140.0 ∘c to ice at -55.0 ∘c? the heat c...
Questions

Mathematics, 24.03.2021 01:10

Health, 24.03.2021 01:10

Mathematics, 24.03.2021 01:10

History, 24.03.2021 01:10

Mathematics, 24.03.2021 01:10

Mathematics, 24.03.2021 01:10

Mathematics, 24.03.2021 01:10



Mathematics, 24.03.2021 01:10



Chemistry, 24.03.2021 01:10

Social Studies, 24.03.2021 01:10



Mathematics, 24.03.2021 01:10



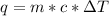
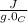 and
and  is change in temperature.
is change in temperature.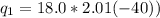
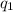 = -1447.2 J
= -1447.2 J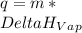
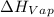 is the enthalpy of vaporization. It's value is 2260 J per gram.
is the enthalpy of vaporization. It's value is 2260 J per gram.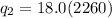
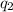 = 40680 J
= 40680 J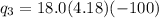
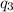 = -7524 J
= -7524 J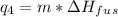
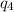 = 18.0(333.55)
= 18.0(333.55)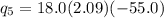
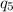 = -2069.1 J
= -2069.1 J


