
Chemistry, 13.07.2019 18:30 adyenamaie02
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphorus and excess oxygen. in part b, you found the amount of product (3.40 mol p2o5 ) formed from the given amount of oxygen and excess phosphorus. now, determine how many moles of p2o5 are produced from the given amounts of phosphorus and oxygen. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
In part a, you found the amount of product (3.80 mol p2o5 ) formed from the given amount of phosphor...
Questions

Mathematics, 20.11.2020 19:50

Computers and Technology, 20.11.2020 19:50

Chemistry, 20.11.2020 19:50


Mathematics, 20.11.2020 19:50



Physics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50



Mathematics, 20.11.2020 19:50


Social Studies, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50




Social Studies, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

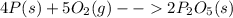
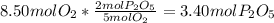
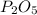 is formed from P (limiting reactant):
is formed from P (limiting reactant):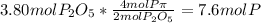
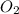 =
= 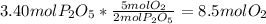
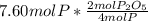
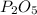 produced from P are
produced from P are 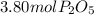
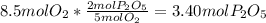
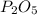 produced will be 3.40 mol
produced will be 3.40 mol

