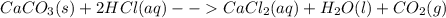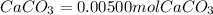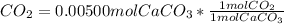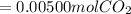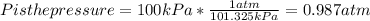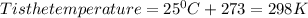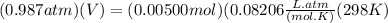Chemistry, 13.07.2019 18:30 auzriannamarie
Chris the chemist works in a laboratory in which the temperature is maintained at a constant 25oc and the pressure is always 100 kpa. chris needs to analyse some calcium carbonate, caco3(s), to determine whether it is pure or has been contaminated. chris will analyse the calcium carbonate by taking a small 0.00500 mole sample and adding hydrochloric acid, hcl(aq), to it until all the calcium carbonate has disappeared and no more carbon dioxide gas, co2(g), is produced. as the gas is produced it will be collected by a water displacement method. the balanced chemical equation for this reaction is known to be: caco3(s) + 2hcl(aq) → cacl2(aq) + co2(g) + h2o(l) if the sample is pure, what volume of carbon dioxide gas will be collected?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Chris the chemist works in a laboratory in which the temperature is maintained at a constant 25oc an...
Questions




Chemistry, 31.08.2020 01:01



Arts, 31.08.2020 01:01

Mathematics, 31.08.2020 01:01


Mathematics, 31.08.2020 01:01

English, 31.08.2020 01:01

Mathematics, 31.08.2020 01:01


Mathematics, 31.08.2020 01:01



Mathematics, 31.08.2020 01:01

Health, 31.08.2020 01:01


Mathematics, 31.08.2020 01:01