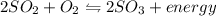Chemistry, 13.07.2019 18:00 Woodsydal2390
Which situation would cause the following equilibrium reaction to decrease the formation of the products? 2so2 (g) + o2 (g) two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2so3 (g) + energy increase the temperature decrease the volume increase the pressure decrease the temperature

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Which situation would cause the following equilibrium reaction to decrease the formation of the prod...
Questions

Mathematics, 12.09.2019 19:30




History, 12.09.2019 20:10



Biology, 12.09.2019 20:10


History, 12.09.2019 20:10

Chemistry, 12.09.2019 20:10


Geography, 12.09.2019 20:10



Mathematics, 12.09.2019 20:10

Mathematics, 12.09.2019 20:10



Chemistry, 12.09.2019 20:10