Chemistry, 13.07.2019 16:30 rerunkle96
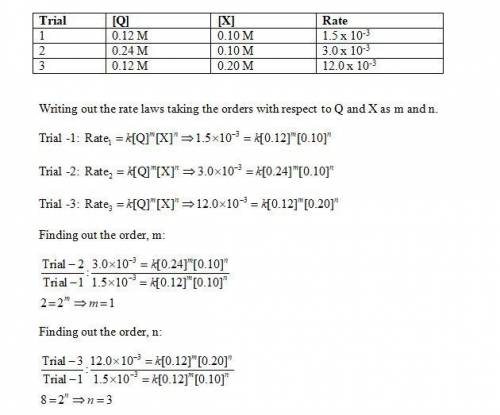
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. q + x yields products trial [q] [x] rate 1 0.12 m 0.10 m 1.5 × 10-3 m/min 2 0.24 m 0.10 m 3.0 × 10-3 m/min 3 0.12 m 0.20 m 12.0 × 10-3 m/min

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

You know the right answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions

Mathematics, 26.05.2021 20:50


Mathematics, 26.05.2021 20:50


English, 26.05.2021 20:50

History, 26.05.2021 20:50



Mathematics, 26.05.2021 20:50

World Languages, 26.05.2021 20:50


Mathematics, 26.05.2021 20:50

English, 26.05.2021 20:50

English, 26.05.2021 20:50

Mathematics, 26.05.2021 20:50

Mathematics, 26.05.2021 20:50


History, 26.05.2021 20:50

Mathematics, 26.05.2021 20:50

Business, 26.05.2021 20:50