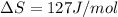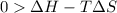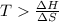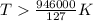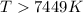Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
At what temperature is the following reaction feasible: hcl(g) + nh3(g) -> nh4cl(s)?
Questions




Mathematics, 12.08.2020 08:01




Chemistry, 12.08.2020 08:01

Physics, 12.08.2020 08:01




Mathematics, 12.08.2020 08:01




Mathematics, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01

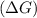 should be negative or
should be negative or 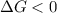 .
.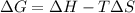
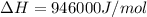 and
and