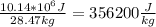Chemistry, 13.07.2019 13:00 fannyrivera321
Aliquid takes 10.14 x 10^6 j of energy to boil 28.47 kg at 298 k. using the lanten heats of vaporization of 5 liquids below, determine what the substance is: acetone: 538,900 j kg-1ammonia: 1,371,000 j kg-1propane: 356, 000 j kg-1methane: 480, 600 j kg-1ethanol: 841,000 j kg-1a) ethanolb) ammoniac) propaned) acetone

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 14:30
Hydrogen has three isotopes 1h, 2h, and 3h. what is the difference between these three isotopes?
Answers: 2
You know the right answer?
Aliquid takes 10.14 x 10^6 j of energy to boil 28.47 kg at 298 k. using the lanten heats of vaporiza...
Questions


English, 06.05.2020 05:10


Mathematics, 06.05.2020 05:10


Spanish, 06.05.2020 05:10


History, 06.05.2020 05:10



History, 06.05.2020 05:10

Mathematics, 06.05.2020 05:10



Biology, 06.05.2020 05:10

English, 06.05.2020 05:10


English, 06.05.2020 05:10


Mathematics, 06.05.2020 05:10