
Chemistry, 13.07.2019 12:30 codeyhatch142
One way to identify a substance is to compare the density of the substance with the density of a known substance. suppose a cube of a silver-colored metal with a volume of 64 cm3 has a mass of 672 g. the density of pure silver is known to be 10.5 g/cm3. based on this information, could the metal be pure silver? a. no. the density is greater than pure silver's. b. no. the density is less than pure silver's. c. yes. the density equals that of pure silver.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

You know the right answer?
One way to identify a substance is to compare the density of the substance with the density of a kn...
Questions


Mathematics, 09.07.2019 01:30

English, 09.07.2019 01:30

History, 09.07.2019 01:30

Physics, 09.07.2019 01:30



Mathematics, 09.07.2019 01:30


Physics, 09.07.2019 01:30

Mathematics, 09.07.2019 01:30


Mathematics, 09.07.2019 01:30


Mathematics, 09.07.2019 01:30

Mathematics, 09.07.2019 01:30

Mathematics, 09.07.2019 01:30


History, 09.07.2019 01:30

Social Studies, 09.07.2019 01:30

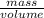 =
= 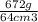 cm³
cm³

