Chemistry, 13.07.2019 09:30 paolaz80045
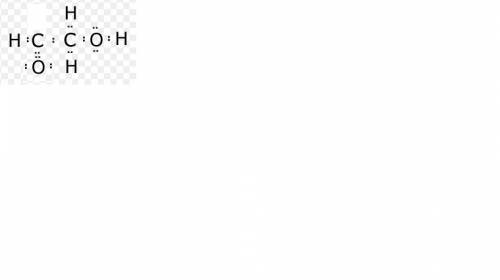
Draw it draw lewis dot structures for each hypothetical molecule shown below, using the correct number of valence electrons for each atom. determine which molecule makes sense because each atom has a complete valence shell and each bond has the correct number of electrons. explain what makes the other molecules nonsensical, considering the num- ber of bonds each type of atom can make

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
Draw it draw lewis dot structures for each hypothetical molecule shown below, using the correct numb...
Questions

Biology, 28.09.2021 17:50


Business, 28.09.2021 17:50


Mathematics, 28.09.2021 17:50

History, 28.09.2021 17:50


Mathematics, 28.09.2021 17:50



History, 28.09.2021 17:50

Mathematics, 28.09.2021 18:00


Mathematics, 28.09.2021 18:00



Mathematics, 28.09.2021 18:00


Mathematics, 28.09.2021 18:00