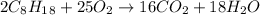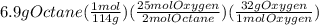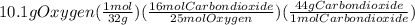Chemistry, 13.07.2019 09:30 cyaransteenberg
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 6.9 g of octane is mixed with 10.1 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

You know the right answer?
Problem page liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon di...
Questions

Mathematics, 17.10.2019 19:00

English, 17.10.2019 19:00

Social Studies, 17.10.2019 19:00


Mathematics, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00

Computers and Technology, 17.10.2019 19:00




Mathematics, 17.10.2019 19:00